2,4-Dichlorotoluene
Structural formula
| Business number | 029P |
|---|---|
| Molecular formula | C7H6Cl2 |
| Molecular weight | 161 |
| label |
2,4-Dichloro-1-methylbenzene, 2,4-Dichlorotoluene (dichlorotoluene), 2,4-Dichloro-1-toluene, 2,4-Dichloro-1-methylbenzene, 2,4-Dichloromethylbenzene, Aromatic halogen derivatives |
Numbering system
CAS number:95-73-8
MDL number:MFCD00000583
EINECS number:202-445-8
RTECS number:XT0730000
BRN number:1931691
PubChem number:24848696
Physical property data
1. Properties: colorless and transparent liquid with pungent odor. [1]
2. Melting point (℃): -13.5[2]
3. Boiling point (℃): 200[3]
4. Relative density (water = 1): 1.25[4]
5. Critical pressure (MPa): 3.59[5]
6. Octanol/water partition coefficient: 4.24[6]
7 .Flash point (℃): 79.44[7]
8. Ignition temperature (℃): >500[8]
9. Explosion upper limit (%): 4.5[9]
10. Explosion lower limit (%): 1.9[10]
11. Solubility: Insoluble in water, miscible in ethanol, ether and benzene. [11]
Toxicological data
1. Acute toxicity: rat oral LD50: 3249mg/kg; mouse oral LD50: 2400mg/kg; guinea pig oral LD50: 5mg/kg;
2. Other multiple dose toxicity : Rat oral TDLo: 6440mg/kg/2W-I; Rat oral TDLo: 28980mg/kg/9W-I;
3. Acute toxicity[ 12] LD50: 2400mg/kg (orally in rats); 2900mg/kg (orally in mice)
4. Irritation No information yet
Ecological data
1. Ecotoxicity[13] LC50: 4.6mg/L (7d) (fish)
2. Biodegradability No data yet
3. Non-biodegradability[14] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 12d (theoretical).
4. Bioconcentration[15] BCF: 1000 (theoretical)
Molecular structure data
1. Molar refractive index: 40.86
2. Molar volume (cm3/mol): 129.6
3. Isotonic specific volume (90.2K ): 316.6
4. Surface tension (dyne/cm): 35.6
5. Polarizability: 16.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecular polarity.��Area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 92.9
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances[17] Strong oxidizing agent
3. Conditions to avoid contact[18] Heating
4. Polymerization Hazards[19] No polymerization
5. Decomposition products[20] Hydrogen chloride
Storage method
1. Storage precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. If this product is exposed to fire, it should be stored in a cool and ventilated place, away from fire and heat sources, and stored separately from oxidants, food, feed, and seeds. Pay attention to keeping the packaging intact. Store and transport according to regulations for flammable materials.
Synthesis method
Production method: Its preparation methods include the following.
① Para-chlorotoluene method
Put para-chlorotoluene and catalyst ZrCl4 into the reactor, pass chlorine gas to carry out chlorination reaction, control the amount of chlorine gas until the reaction is completed, and stop reaction, the obtained reactant contains 85.1% of 2,4-dichlorotoluene. If FeCl3 is used as the catalyst to perform the chlorination reaction at 10-15°C until the relative density of the solution is 1.025, the product will contain 2,4-dichlorotoluene and 3,4-dichlorotoluene, the mass ratio of the two components is 100:30. After chlorination is completed, wash with water until neutral, and treat it with 10% NaOH solution at 100-110°C to remove other impurities. The treated chloride is distilled and separated in a high-efficiency distillation tower (2,4-dichloro Toluene b.p.200℃, 3,4-dichlorotoluene b.p.207℃). The yields of 2,4-dichlorotoluene and 3,4-dichlorotoluene were 64.4% and 19.8% respectively.
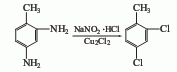
②O-chlorotoluene method
O- Chlorotoluene is chlorinated using sulfuryl chloride as the chlorinating agent at 142-196°C. The products include 2,4-dichlorotoluene and 2,3-dichlorotoluene, as well as unreacted raw materials. Its composition They are 55%, 6% and 39% respectively. After distillation (2,4-dichlorotoluene b.p. 200℃, 2,3-dichlorotoluene b.p. 207~208℃, o-chlorotoluene b.p. 157~159℃), 2,4-dichlorotoluene is separated.
③O-nitrotoluene method
O-nitrotoluene is chlorinated in the presence of FeCl3 catalyst at 35~40℃. When the relative density of the reactants reaches 1.320(15 ℃), wash the material until neutral. The reactants include 15% of raw materials, 49% of 2-chloro-6-nitrotoluene, 21% of 4-chloro-2-nitrotoluene, and 15% of polychlorides. After distillation and crystallization, 2-chloro-6-nitrotoluene and 4-chloro-2-nitrotoluene were obtained, with yields of 50% and more than 30% respectively. 4-chloro-2-nitrotoluene was added Hydrogen reduction reaction and steam distillation yield 4-chloro-2-aminotoluene, which is then diazotized and CH2Cl2 is added for Sandmeyer reaction to yield 2,4 -Dichlorotoluene. This method is used to produce 4-chloro-2-nitrotoluene, a by-product of 2-chloro-6-nitrotoluene (used as an intermediate for the herbicide quinclorac).
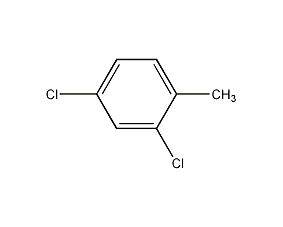
④2,4-Diaminotoluene method
2,4-Diaminotoluene is diazotized in the presence of NaNO2 and hydrochloric acid, and then in Cu2 Sandmeyer reaction is carried out in the presence of Cl2 to obtain 2,4-dichlorotoluene.
⑤3-Chloro-4-methylaniline method
Add 3-chloro-4-methylaniline and hydrochloric acid into the reaction kettle, and add NaNO2The aqueous solution is added dropwise within 2 to 3 hours to carry out the diazotization reaction, and then the diazotization solution is added dropwise to the diazotization solution containing Cu2Cl2 at 2 to 5°C. The hydrochloric acid solution is subjected to Sandmeyer reaction to obtain 2,4-dichlorotoluene.
Among the above methods, p-chlorotoluene is used The chlorides produced as raw materials with o-chlorotoluene contain many impurities and have similar boiling points. Only by using a high-efficiency distillation tower to obtain more than 98% of 2,4-dichlorotoluene can we obtain 2,4-dichlorotoluene. These two methods are difficult to operate and require high equipment investment costs. The 2,4-diaminotoluene method is not suitable for industrialization, but the basic principles of preparing 2,4-dichlorotoluene by the o-nitrotoluene method and the 3-chloro-4-methylaniline method are the same, both of which require diazotization and Sandmeyer Reactive, there is a disadvantage of more waste water. The o-nitrotoluene method co-produces 2-chloro-6-nitrotoluene, which is further reduced to obtain 2-chloro-6-aminotoluene, which is an important intermediate for the production of the herbicide quinclorac.
Purpose
1. Pesticide intermediates, used to manufacture 2,4-dichlorobenzyl chloride and 2,4-dichlorobenzoyl chloride. It is used in the pharmaceutical industry to manufacture the antimalarial drug Adipine.
2. Used as a solvent for pharmaceuticals and organic synthesis. [22]
extended-reading:https://www.newtopchem.com/archives/40320
extended-reading:https://www.newtopchem.com/archives/44134
extended-reading:https://www.cyclohexylamine.net/organic-bismuth-catalyst-dabco-mb20-dabco-mb20/
extended-reading:https://www.cyclohexylamine.net/low-odor-tertiary-amine-catalyst-dabco-low-odor-tertiary-amine-catalyst/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyltin-oxide-Ultra-Pure-818-08-6-CAS818-08-6-Dibutyloxotin.pdf
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Butyl-tin-triisooctoate-CAS23850-94-4-FASCAT9102-catalyst.pdf
extended-reading:https://www.newtopchem.com/archives/40491
extended-reading:https://www.morpholine.org/tris3-dimethylaminopropylamine/
extended-reading:https://www.cyclohexylamine.net/dabco-ne1070-gel-type-low-odor-catalyst/
extended-reading:https://www.morpholine.org/bis3-dimethylaminopropylamino-2-propanol/


