Spironolactone sterol
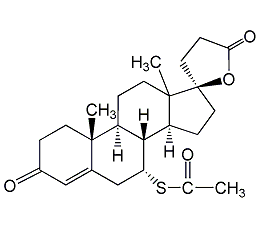
Structural formula
| Business number | 015D |
|---|---|
| Molecular formula | C24H32O4S |
| Molecular weight | 416.57 |
| label |
(7α,17α)-7-(acetylmercapto)-17-hydroxy-3-oxopregnant-4-en-21-carboxylic acid γ-lactone, spironolactone, Spironolactone |
Numbering system
CAS number:52-01-7
MDL number:MFCD00082250
EINECS number:200-133-6
RTECS number:TU4725000
BRN number:None
PubChem number:24277736
Physical property data
1. Properties: White or off-white fine crystalline powder with a slight mercaptan odor and a slightly bitter taste.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 202~208
5. Boiling point (ºC, normal pressure): 182~184
6. Boiling point (ºC, 5.2kPa) : Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): -37
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12 . Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
p>
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in chloroform, benzene, Ethyl acetate, soluble in ethanol, insoluble in water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 112.68
2. Molar volume (cm3/mol): 335.7
3. Isotonic specific volume (90.2K ): 896.8
4. Surface tension (dyne/cm): 50.8
5. Polarizability (10-24cm3): 44.67
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 10
6. Topological molecular polar surface area (TPSA): 60.4
p>
7. Number of heavy atoms�: 29
8. Surface charge: 0
9. Complexity: 818
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 7
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
It can be synthesized from ethinyl androdiol through Grignard reaction, carboxylation, salt formation, hydrogenation, lactonization, oxidation, elimination, addition and other steps.
Purpose
It is a diuretic and has anti-aldosterone effect. It is used to treat liver disease and nephrotic edema related to excessive aldosterol. Preparations include tablets and capsules.
extended-reading:https://www.bdmaee.net/fascat2004-catalyst-anhydrous-tin-dichloride-arkema-pmc/extended-reading:https://www.bdmaee.net/dabco-ne1080-catalyst-cas31506-44-2-evonik-germany/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Tributyltin-chloride-CAS1461-22-9-tri-n-butyltin-chloride.pdfextended-reading:https://www.bdmaee.net/fascat4102-catalyst-monobutyl-tin-triisooctanoate-cas-23850-94-4/extended-reading:https://www.bdmaee.net/nt-cat-t16-catalyst-cas10102-43-9-newtopchem/extended-reading:https://www.newtopchem.com/archives/44944extended-reading:https://www.bdmaee.net/dabco-ncm-catalyst-cas110-18-9-evonik-germany/extended-reading:https://www.bdmaee.net/dibutyltin-oxide-ultra-pure-818-08-6-cas818-08-6-dibutyloxotin/extended-reading:https://www.newtopchem.com/archives/516extended-reading:https://www.bdmaee.net/organic-mercury-replacement-catalyst-nt-cat-e-at/


