2,5-Dimethylaniline
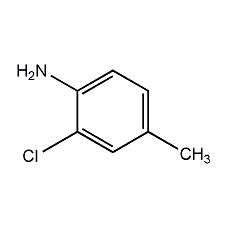
Structural formula
| Business number | 029U |
|---|---|
| Molecular formula | C8H11N |
| Molecular weight | 121.18 |
| label |
1-amino-2,5-xylene, p-dimethylaniline, 2,5-Dimethylaniline, 1-Amino-2,5-dimethylbenzene, 2,5-Dimethylbenzenamine |
Numbering system
CAS number:95-78-3
MDL number:MFCD00007743
EINECS number:202-451-0
RTECS number:ZE9100000
BRN number:2205178
PubChem number:24846569
Physical property data
1. Properties: Colorless or light yellow oily liquid, which forms crystals at low temperatures. [1]
2. Melting point (℃): 15.5[2]
3. Boiling point (℃): 214 ~217[3]
4. Relative density (water = 1): 0.98[4]
5. Relative Vapor density (air=1): 4.18[5]
6. Saturated vapor pressure (kPa): 1.33 (97℃)[6]
7. Octanol/water partition coefficient: 1.83~2.21[7]
8. Flash point (℃): 93.89[8 ]
9. Explosion upper limit (%): No information yet
10. Explosion lower limit (%): 1.5[9]
11. Solubility: insoluble in water, slightly soluble in alcohol, soluble in ether and chloroform. [10]
Toxicological data
1. Acute toxicity: rat oral LD50: 1297mg/kg; mouse oral LD50: 841mg/kg;
2. Other multiple dose toxicity: rat oral TDLo: 13300mg/ kg/4W-I;
3. Mutagenicity
Mutation of microorganism Salmonella typhimurium: 10μmol/plate;
DNA synthesis in rat liver: 10μmol /L;
Oral DNA inhibition in mice: 200 mg/kg;
4. Acute toxicity [11] LD50: 1120mg/kg (rat oral)
5. Irritation No information available
Ecological data
1. Ecotoxicity[12] IC50: 5mg/L (72h) (algae)
2. Biodegradability No data yet
3. Non-biodegradability[13] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 2h (theoretical).
4. Other harmful effects [14] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 40.20
2. Molar volume (cm3/mol): 119.9
3. Isotonic specific volume (90.2K ): 306.6
4. Surface tension (dyne/cm): 42.7
5. Polarizability (10-24cm3): 15.94
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5.Number of tautomers: None
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 90.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The color gradually becomes darker in light and air. Can evaporate with steam.
2. Stability[15] Stable
3. Incompatible substances[16] Acids, acid chlorides, acid anhydrides, strong oxidants, chloroform, halogens
4. Conditions to avoid contact [17] Heating
5. Polymerization hazard[18] No polymerization
Storage method
Storage Precautions[19] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, halogens, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It is produced by nitration and reduction of p-xylene.
Purpose
1. Used in pharmaceutical and dye industries. [20]
extended-reading:https://www.bdmaee.net/pc-cat-dmcha-catalyst/extended-reading:https://www.newtopchem.com/archives/1888extended-reading:https://www.cyclohexylamine.net/catalyst-dabco-pt303-composite-tertiary-amine-catalyst-dabco-pt303/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Butyl-tin-triisooctoate-CAS23850-94-4-FASCAT9102-catalyst.pdfextended-reading:https://www.cyclohexylamine.net/pc-cat-nmm-addocat-101-tertiary-amine-catalyst-nmm/extended-reading:https://www.bdmaee.net/methyl-tin-maleate-powder-c6h8o4sn-methyl-tin-maleate/extended-reading:https://www.newtopchem.com/archives/38895extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/1-1.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/22-1.jpgextended-reading:https://www.bdmaee.net/niax-potassium-acetate-trimer-catalyst-momentive/


