4-nitroquinoline N-oxide
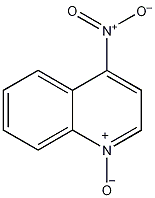
Structural formula
| Business number | 017S |
|---|---|
| Molecular formula | C9H6N2O3 |
| Molecular weight | 190.16 |
| label |
1-Oxo-4-nitroquinoline, 4-Nitroquinoline N-oxide |
Numbering system
CAS number:56-57-5
MDL number:MFCD00006738
EINECS number:200-281-1
RTECS number:VC2100000
BRN number:165756
PubChem number:24897854
Physical property data
1. Character: light yellow-brown flake Or needle crystals.
2. Density ( g/mL,25/4℃) : Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point ( ºC): 151~153 ℃(156~157℃).
5. Boiling point ( ºC,Normal pressure): Undetermined
6. Boiling point ( ºC,5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not OK
11. Vapor pressure (kPa,25ºC): Undetermined
12. saturated vapor pressure (kPa,60ºC): Undetermined
13. heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Oil and water (octanol/Water) partition coefficient pair Value: Undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Not OK
19. Solubility: Undetermined.
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 71.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 229
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be kept sealed.
Synthesis method
Nitration of quinoline with sulfuric acid and nitric acid-N-Oxide.
Purpose
For cancer research. Used in organic synthesis. An effective preservative��But it has carcinogenic effects.
extended-reading:https:////www.bdmaee.net/wp-content/uploads/2022/08/-MP601-delayed-equilibrium-catalyst–MP601-catalyst.pdfextended-reading:https://www.bdmaee.net/nt-cat-t1-catalyst-cas77-58-7-newtopchem/extended-reading:https://www.bdmaee.net/niax-ef-708-foaming-catalyst-momentive/extended-reading:https://www.cyclohexylamine.net/dimethyltin-dioctanoate-cas-68928-76-7/extended-reading:https://www.bdmaee.net/fentacat-f13-catalyst-cas80284-38-9-solvay/extended-reading:https://www.newtopchem.com/archives/44710extended-reading:https://www.newtopchem.com/archives/category/products/page/19extended-reading:https://www.cyclohexylamine.net/methyl-tin-maleate-powder-methyltin-maleate/extended-reading:https://www.bdmaee.net/nt-cat-a-1-catalyst-cas3033-62-3-newtopchem/extended-reading:https://www.newtopchem.com/archives/category/products/page/99


