carbon tetrachloride
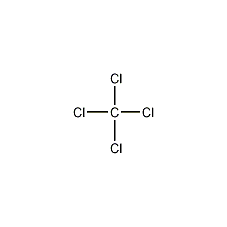
Structural formula
| Business number | 017B |
|---|---|
| Molecular formula | CCl4 |
| Molecular weight | 153.82 |
| label |
Tetrachloromethane, Perchloromethane, Tetrachloromethane, Perchloromethane, Benzimoform, Aliphatic halogenated derivatives |
Numbering system
CAS number:56-23-5
MDL number:MFCD00000785
EINECS number:203-453-4
RTECS number:203-453-4
BRN number:1098295
PubChem number:24857387
Physical property data
1. Properties: Colorless, transparent and volatile liquid with a special aromatic odor.
2. Boiling point (ºC, 101.3kPa): 76.72
3. Melting point (ºC): -22.92
4. Relative density (g/mL, 20/4ºC): 1.5942
5. Refractive index (n20ºC): 1.4604
6. Viscosity (mPa·s, 20ºC): 0.965
7. Heat of evaporation (KJ/mol, b.p.): 29.982
8. Heat of fusion (KJ/mol): 2.433
9. Heat of formation (KJ/mol, 25ºC, liquid): 135.5
10. Heat of combustion (KJ/mol, 25ºC, liquid): 258.24
11. Specific heat capacity (KJ/(kg·K), 20ºC): 0.866
12. Critical temperature (ºC): 283.15
13. Critical pressure (MPa): 4.56
14. Boiling point rise constant: 4.88
15 . Conductivity (S/m, 18ºC): 4×10-18
16. Volume expansion coefficient (K-1, 20ºC): 0.00127
17. Solubility: Miscible with most organic solvents such as water, alcohol, ether, petroleum ether, naphtha, glacial acetic acid, carbon disulfide, chlorinated hydrocarbons, etc.
18. Relative density (25℃, 4℃): 1.5846
19. Refractive index at room temperature (n25): 1.4573
20. Vapor pressure (kPa, 20ºC): 11.9102
21. Critical density (g·cm-3): 0.557
22. Critical Volume (cm3·mol-1): 276
23. Critical compression factor: 0.272
24. Eccentricity factor :0.193
25. Lennard-Jones parameter (A): 7.949
26. Lennard-Jones parameter (K): 633.6
27. Solubility parameter (J·cm-3)0.5:17.577
28. van der Waals area (cm2·mol-1): 7.280×109
29. van der Waals volume (cm3·mol– 1): 52.300
30.p; Gas phase standard claims heat (enthalpy) (kJ·mol-1): -95.8
31. Gas phase standard entropy (J·mol-1·K-1): 310.02
32. Gas phase standard free energy of formation (kJ·mol-1): -53.5
33. Gas phase standard hot melt (J·mol-1·K-1): 83.43
34. Liquid phase standard claims heat (Enthalpy) (kJ·mol-1): -128.41
35. Liquid phase standard entropy (J·mol-1·K-1): 216.19
36. Liquid phase standard free energy of formation (kJ·mol-1): -60.50
37 . Liquid phase standard hot melt (J·mol-1·K-1): 131.4
Toxicological data
1. Acute toxicity[1]
LD50: 2350mg/kg (rat oral); 5070mg/kg (rat oral Skin)
LC50: 50400mg/m3 (rat inhalation, 4h)
2. Irritation [2]
Rabbit transdermal: 4 mg, mild irritation.
Rabbit eye: 500mg (24h), mild irritation.
3. Subacute and chronic toxicity [3] Long-term low-dose exposure to this product mainly damages the liver and kidneys.
4. Mutagenicity [4] Microbial mutagenicity: Salmonella typhimurium 20μl/L. DNA damage: mouse oral administration: 335 μmol/kg. Sex chromosome deletion and non-disjunction: hamster lung 1600 μmol/L. Unprogrammed DNA synthesis: 100mg/kg orally administered to mice. DNA inhibition: 2g/kg orally administered to mice.
5. Teratogenicity[5] Inhalation of the lowest toxic dose (TCLo) 300ppm (7h) in rats 6 to 15 days after pregnancy can cause musculoskeletal damage Phylogenetic abnormalities. The lowest toxic dose (TCLo) of 2384 mg/kg was administered parenterally to rats on the 18th day after pregnancy, causing developmental malformation of the hepatobiliary system.
6. Carcinogenicity [6] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
7. Others[7] The lowest oral toxic dose in rats (TDLo): 2g/kg (gestation 7~8d), causing implantation Mortality rate increases after admission. The lowest oral toxic dose (TDLo) in rats was 3691 mg/kg (male, 10 days), causing abnormalities in the testicles, epididymis and vas deferens.
Ecological data
1. Ecotoxicity[8]
LC50: 27~125mg/L (96h) (bluegill sunfish); 20.8~ 41.4mg/L (96h) (fathead minnow); 45mg/L (96h) (green algae)
IC50: 600mg/L (72h) (algae)
2. Biodegradability[9]
Aerobic biodegradation (h): 4032~8640
Anaerobic biodegradation ( h): 168~672
3. Non-biodegradability[10]
Photooxidation half-life in air (h ): 1.60×104~1.60×105
First-level hydrolysis half-life (h): 7000
4. Other harmful effects [11] Carbon tetrachloride is a highly accumulating substance that can accumulate in the livers of mammals and can cause liver cancer in salmon. This substance has an ozone depletion potential (ODP) of 1.1 and is extremely destructive to the atmospheric ozone layer.
Molecular structure data
1. Molar refractive index: 26.04
2. Molar volume (cm3/mol): 90.6
3. Isotonic specific volume (90.2K ): 220.7
4. Surface tension (dyne/cm): 35.2
5. Polarizability (10-24cm3): 10.32
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 19.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[12] Stable
2. Incompatible substances[13] Active metal powder, strong oxidant
3. Conditions to avoid contact[14] Humid air, light
4. Hazards of aggregation[15] No aggregation
Storage method
Storage Notes[16] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 32°C and the relative humidity does not exceed 80%. Keep container tightly sealed. They should be stored separately from oxidants, active metal powders, and food chemicals, and avoid mixed storage. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Methane thermal chlorination method: Methane and chlorine are mixed, and a thermal chlorination reaction occurs at 400 to 430°C to obtain crude product and by-product hydrochloric acid. The crude product is neutralized, dried, and distilled to purify to obtain the finished product. 
In addition to hydrochloric acid, the by-products include chloroform, tetrachloroethylene and hexachloroethane, all of which can be recycled and sold.
2 .Carbon disulfide method uses iron as a catalyst to react chlorine and carbon disulfide at 90 to 100°C. The reaction product is fractionated, neutralized, and distilled to obtain the finished product. This method requires less investment and the product is easy to purify, but the cost is high and the equipment is seriously corroded. 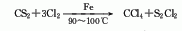
3. Methane oxychlorination method has high chlorine utilization rate. No pollution from hydrogen chloride and waste halogenated hydrocarbons.
4. The high-pressure chlorination method avoids the production of tetrachlorethylene. 5. The methanol hydrochlorination method has good quality and high economic benefits. , both dichloromethane and chloroform can be co-produced with tetrachloromethane
6. Combine 100L industrial carbon tetrachloride, 15L 95% ethanol, 1.5g 42% sodium hydroxide and lead acetate. Mix, stir and heat to boil until the sulfide content test is passed. Then add water and wash it for more than three times. Discard the water layer and add concentrated sulfuric acid (10kg of sulfuric acid per 100kg of carbon tetrachloride). Stir and pickle until the sulfuric acid test passes. Then wash with water. After three times, add 5% sodium hydroxide solution, stir thoroughly and let it stand. Discard the water layer and repeat alkali washing until the acidity is qualified. Add an appropriate amount of anhydrous calcium chloride and a small amount of anhydrous to the carbon tetrachloride that has passed the alkali washing. Sodium carbonate, let it stand fully, filter out the desiccant, distill it under normal pressure, and collect the 76-77.5°C fraction, which is the finished product.
Purpose
1. Mainly used as excellent solvents, dry cleaning agents, fire extinguishing agents, refrigerants, spice leaching agents and pesticides, etc.
2. It can be used to synthesize monomers of Freon, nylon 7, and nylon 9; it can also be used to prepare chloroform and drugs; it can be used as a lubricant in metal cutting.
3. Used as analytical reagents, such as standards and solvents for measuring chlorine. Also used as a cleaning agent, extraction solvent, and pesticide.
4. Used in organic synthesis as solvent, refrigerant, chlorinating agent for organic matter, leaching agent for spices, degreasing agent for fibers, etc. [17]
extended-reading:https://www.newtopchem.com/archives/698
extended-reading:https://www.cyclohexylamine.net/dabco-ncm-polyester-sponge-catalyst-dabco-ncm/
extended-reading:https://www.bdmaee.net/cas-77-58-7/
extended-reading:https://www.newtopchem.com/archives/40296
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/2.jpg
extended-reading:https://www.bdmaee.net/catalyst-9727-9727/
extended-reading:https://www.morpholine.org/non-emissive-polyurethane-catalyst-dabco-ne1060-catalyst/
extended-reading:https://www.newtopchem.com/archives/39847
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dioctyl-dimaleate-di-n-octyl-tin-CAS33568-99-9-Dioctyl-dimaleate-di-n-octyl-tin.pdf
extended-reading:https://www.cyclohexylamine.net/catalyst-9727-polyurethane-catalyst-9727/


