Dibromomethane
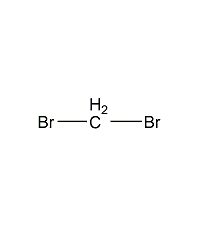
Structural formula
| Business number | 01HQ |
|---|---|
| Molecular formula | CH2Br2 |
| Molecular weight | 173 |
| label |
Methyl bromide, methylene bromide, methylene dibromide, Dibromomethylene, Methylene dibromide, Methylene bromide, Aliphatic halogenated derivatives |
Numbering system
CAS number:74-95-3
MDL number:MFCD00000168
EINECS number:200-824-2
RTECS number:PA7350000
BRN number:969143
PubChem number:24893828
Physical property data
1. Properties: colorless transparent liquid [9]
2. Melting point (℃): -52.5[10]
3. Boiling point (℃): 96~98[11]
4. Relative density (water=1): 2.48[12]
5. Relative vapor density (air=1): 6.05[13]
6. Saturated vapor pressure (kPa): 5 (20℃)[14]
7. Critical temperature (℃): 309.8[15]
8. Critical pressure (MPa): 7.15[16]
9. Octanol/water partition coefficient: 1.7[17]
10. Solubility: Slightly soluble in water, miscible in ethanol, ether, acetone, and chloroform. [18]
11. Viscosity (mPa·s, 20ºC): 10.2
12. Heat of evaporation (KJ/kg, b.p.): 36.46
13. Specific heat capacity (KJ/(kg·K), 20ºC): 0.66
14. Thermal conductivity (W/(m·K), 20ºC): 0.1026
15. Relative density (20℃, 4℃): 1.4970
16. Relative density (25℃, 4℃): 1.4842
17. Eccentricity factor: 0.210
18. Solubility parameter (J·cm-3)0.5: 22.344
19. van der Waals area ( cm2·mol-1): 5.530×109
20. van der Waals volume (cm3·mol-1): 39.430
21. Liquid phase standard hot melt (J·mol-1·K-1): 105.1
22. Gas phase standard entropy (J·mol-1·K-1): 293.39
23. Gas phase standard hot melt (J·mol-1·K-1): 54.55
Toxicological data
1. Acute toxicity: rat oral LD50: 108 mg/kg; rat inhalation LC50: 40 gm/m3/2H; mouse subcutaneous injection LD50: 3738 mg/kg; rabbit oral LD50: 1 mg/ kg; Rabbit Administration onto the skin LD50: >4 mg/kg; Rabbit rectal LDLo: 5 mg/kg;
2. Mutagenicity: Microbial testing system for Salmonella gene mutations: 100 ng/plate; Salmonella Gene mutation microbial test system: 10 ug/plate; Hamster lung cytogenetic analysis test system: 1 umol/L;
3. Acute toxicity[19]
LD50: 1000mg/kg (rat oral)
LC50: 40000mg/m3 ( Rat inhalation, 2h)
4. Irritation No data available
5. Subacute and chronic toxicity [20] Rats inhaled 1000ppm 54 times, causing liver and kidney damage, and some animals died.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[21]
Aerobic biodegradation (h): 168~672
Anaerobic biodegradation (h): 672~2688
3 .Non-biodegradability[22]
Photooxidation half-life in air (h): 851~8510
First-order hydrolysis half-life (h): 1.60×106
Molecular structure data
1. Molar refractive index: 19.04
2. Molar volume (cm3/mol): 74.7
3. Isotonic specific volume (90.2K ): 163.8
4. Surface tension (dyne/cm): 23.1
5. Polarizability (10-24cm3): 7.55
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[23] Stable
2. Incompatible substances[24] Strong oxidants, aluminum, magnesium
3. Conditions to avoid contact[25] Light and heat
4. Polymerization hazard[26] No polymerization
5. Decomposition products[27] Hydrogen bromide
Storage method
Storage Precautions[28] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, aluminum, metal powders, and food chemicals, and avoid mixed storage. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Bromomethane method: First react arsenic trioxide with liquid alkali to prepare sodium arsenite solution. Heat the sodium arsenite solution to 65°C, and slowly add bromoform while stirring. After all additions, continue to stir and reflux for 4 hours to complete the reaction. Pour the reactants into 5 to 6 times of water, suck out the oil, and carry out fractionation. Then wash with water until neutral, use calcium chloride to dehydrate and distill, to obtain the product. ![]()
2. The dichloromethane method is still used in industry It is prepared by reacting anhydrous CH2CL2 with HBr under the catalysis of anhydrous ALBr3, and also produces chlorobromomethane.
3. The reaction formula of bromochloromethane hydrogen bromide method is as follows:
Purpose
1. Organic synthetic raw materials. It can be used as a solvent, refrigerant, flame retardant and anti-knock component; used in medicine as a disinfectant and sedative; and also used in the pesticide myclobutanil and other organic synthesis.
2. Dibromomethane can participate in nucleophilic substitution reactions, and its reactivity is between dichloromethane and diiodomethane, and is usually weaker than ordinary alkyl bromide reagents.
Dibromomethane is an effective methylenecarboxaldehyde-forming reagent, so the reactions it participates in are usually irreversible. Not only can it form cis-carboxylic aldehydes, but it can also react with sugar compounds to form stable trans-carboxylic aldehydes (Formula 1)[1]. Due to the presence of hydroxyl groups in the reaction substrate, the reaction can be carried out in polar solvents such as water, but requires the participation of phase transfer reagents such as tetrabutylammonium bromide.
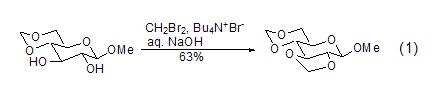
Similar reactions can also use other Phase transfer catalysts such as alkyltrimethylammonium are used to achieve the methylene carboxyformylation reaction of catechol (formula 2)[2] and the monosulfide carboxyformylation reaction of mercaptophenol (formula 3)[3].
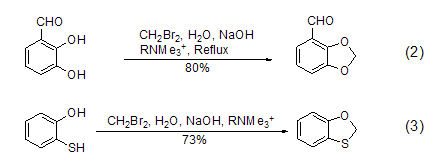
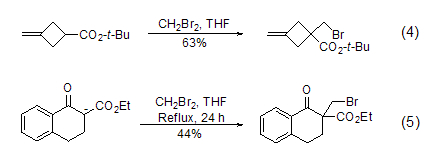
Dibromomethane can also be used in alkenes Monobromination of alcoholic anions. Since enol anions usually produce high steric hindrance, disubstitution reactions with dibromomethane will not occur. Typical examples include the bromomethylation reaction of cyclobutane carboxylate (Formula 4) [4] and tetralone carboxylate (Formula 5) [5] sup>.
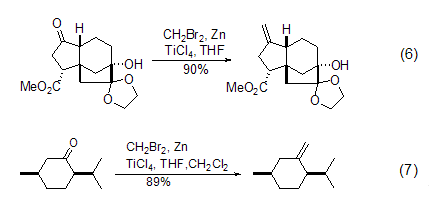
Dibromomethane with Zn and TiCl4 can convert the carbonyl group C=O into C=CH2 with high yield under mild conditions. It is an effective methylene reagent for carbonyl compounds. . The biggest advantage of this mixed reagent compared with the traditional Wittig reagent is that it does not cause the enolization reaction of ketones, so it is very widely used, such as the synthesis of natural products (formula 6)[6] and ordinary ChiralityConversion of reagent (Formula 7)[7].
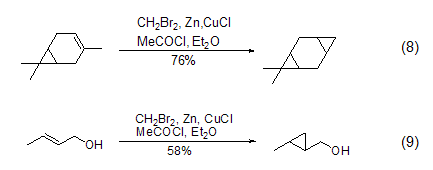
Dibromomethane can also replace diiodo Methane participates in the cyclopropanation reaction of olefins (Formula 8, Formula 9)[8]. This reaction requires the participation of metal Zn, and the activity of Zn will greatly affect the yield of the reaction. Usually, cuprous chloride or acetyl chloride is added to activate metal Zn, and then the reaction intermediate (bromomethyl)zinc bromide is formed to realize the cyclopropanation reaction.
3. Used in organic synthesis, Used as a solvent. [29]
extended-reading:https://www.newtopchem.com/archives/44151
extended-reading:https://www.newtopchem.com/archives/595
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT4350-catalyst-FASCAT-4350.pdf
extended-reading:https://www.newtopchem.com/archives/category/products/page/46
extended-reading:https://www.bdmaee.net/catalyst-dabco-bx405-bx405-polyurethane-catalyst-dabco-bx405/
extended-reading:https://www.bdmaee.net/anhydrous-tin-tetrachloride-2/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2019/10/NEWTOP2.jpg
extended-reading:https://www.newtopchem.com/archives/44594
extended-reading:https://www.cyclohexylamine.net/low-odor-amine-catalyst-bx405-low-odor-strong-gel-catalyst-bx405/
extended-reading:https://www.newtopchem.com/archives/76


