Indomethacin
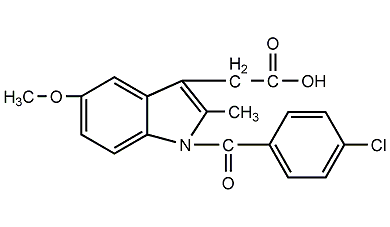
Structural formula
| Business number | 0164 |
|---|---|
| Molecular formula | C19H16ClNO4 |
| Molecular weight | 357.79 |
| label |
indomethacin, anti-inflammatory indoleic acid, 1-p-Chlorobenzoyl-5-methoxy-2-methylindoleacetic acid, Indenaxin, 1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic acid |
Numbering system
CAS number:53-86-1
MDL number:MFCD00057095
EINECS number:200-186-5
RTECS number:NL3500000
BRN number:497341
PubChem number:24278173
Physical property data
1. Character:White crystal. Sensitive to light.
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC):155℃(162℃)
5. Boiling point (ºC,Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive index: undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure (kPa,60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of water) partition coefficient: Undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility:Decomposes when encountering strong alkali. Soluble in ethanol, ether, acetone and castor oil, almost insoluble in water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 94.59
2. Molar volume (m3/mol) :269.5
3. isotonic ratio(90.2K):707.6
4. Surface Tension(dyne/cm):47.4
5. Polarizability(10-24cm3):37.49
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 68.5
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 506
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed in4Preserve dry.
Synthesis method
P-aminoanisole undergoes diazotization, reduction, acidification, hydrolysis, and cyclization to obtain indomethacin.
Purpose
For biochemical research. Biosynthesis of prostaglandins. The It is an anti-inflammatory product with obvious antipyretic effects. It is mainly used for rheumatic arthritis, ankylosing spondylitis, osteoarthritis, etc. that are intolerant to salicylic acid drugs or have insignificant efficacy.
extended-reading:https://www.cyclohexylamine.net/category/product/page/33/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/35.jpg
extended-reading:https://www.bdmaee.net/nt-cat-ea-104-catalyst-cas10027-41-9-newtopchem/
extended-reading:https://www.newtopchem.com/archives/45177
extended-reading:https://www.newtopchem.com/archives/category/products/page/141
extended-reading:https://www.bdmaee.net/22-dimorpholinodiethylether-2/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/potassium-acetate-CAS-127-08-2-Potassium.pdf
extended-reading:https://www.bdmaee.net/tmg-nnn%e2%80%b2n%e2%80%b2-tetramethylguanidine-cas80-70-6/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/23.jpg
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-XD-102–amine-catalyst-amine-catalyst.pdf


