n-tert-butyl-2-benzothiazole sulfinamide
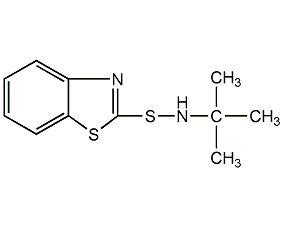
structural formula
| physical competition number | 028y |
|---|---|
| molecular formula | c11h14n2s2 |
| molecular weight | 238.38 |
| label |
accelerator ns, n-(1,1-dimethylethyl)-2-benzothiazole sulfinamide, n-tert-butyl-2-benzothiazole sulfenamide, accelerator tbbs, accelerator ns, n-(1,1 – dimethyl-ethyl) -2 – benzothiazolyl asia sulfonamide, n-tert-butyl-2-benzothiazolyl sulfenamide, accelerator tbbs, slow-acting sulfenamide accelerator |
numbering system
cas number:95-31-8
mdl number:mfcd00022873
einecs number:202-409-1
rtecs number:dl6200000
brn number:158370
pubchem id:none
physical property data
1. properties: the industrial product is light yellow or tan powder.
2. relative density (g/ml, 20℃): 1.26-1.32
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 105-110
5. boiling point (ºc, normal pressure): undetermined
6. boiling point (ºc, kpa): undetermined determined
7. refractive index: undetermined
8. flash point (ºc): 165
9. specific rotation (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (mmhg, ºc): undetermined
12. saturated vapor pressure (kpa, ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) distribution coefficient: undetermined
17. explosion upper limit (%, v/v): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: easily soluble in benzene, methylene chloride, tetrahydrofuran carbon chloride, ethyl acetate, acetone, ethanol, soluble in gasoline, insoluble in water, dilute acid, dilute alkali solution.
toxicological data
1. acute toxicity: rat oral ldlo: 7940mg/kg; mouse abdominal ld50: 5mg/kg; mouse intravenous ld50: 180mg/kg; rabbit skin contact ld50: >7940mg/kg;
2. mutagenicity
mouse lymphocyte mutation: 40mg/l;
rat embryonic morphological transformation: 35mg/l;
ecological data
slightly harmful to water.
molecular structure data
1. molar refractive index: 70.43
2. molar volume (cm3/mol): 194.9
3. isotonic specific volume (90.2k ): 526.1
4. surface tension (dyne/cm): 53.0
5. polarizability (10-24cm3): 27.92
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 4
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 78.5
7. number of heavy atoms: 15
8. surface charge: 0
9. complexity: 215
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
avoid contact with oxides.
storage method
save sealed in a cool, dry place away from direct sunlight. make sure the workspace has good ventilation. keep sealed. keep away from sources of fire and store away from oxidizing agents.
synthesis method
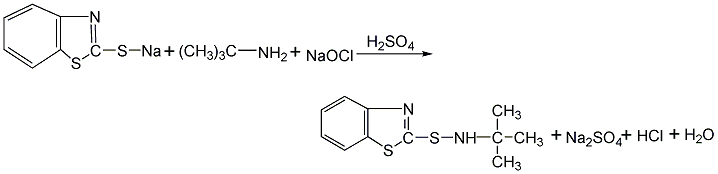
by2-thiobenzothiazole (acceleratorm) obtained by reacting sodium salt with tert-butylamine. to0.5molof13%acceleratorm slowly added to the sodium salt solution0.75moltertiary butylamine, add after half an hour0.36molof25%sulfuric acid solution, in45-50℃ reaction0.5h. again2h<span style="font-family:宋体;font-size 0.6mol15%sodium hypochlorite. after the reaction, cool, filter and wash, 50 products dried below ℃.
purpose
can be used for the after-effects of reclaimed rubber of natural rubber, butadiene rubber, isoprene rubber, styrene-butadiene rubber and natural rubber sex enhancer. this product has the advantages of excellent scorch resistance and short vulcanization time, that is, there is no danger of scorch at the operating temperature, but it has a strong promotion effect at the vulcanization temperature, especially in natural rubber, which has greater aftereffects. this product requires zinc oxide and stearic acid, and can also be activated by thiuram, dithiocarbamates, aldehydeamines, guanidine accelerators and acidic substances. this product has slight contamination and almost no discoloration. it can be used to make light-colored or bright-colored rubber products, mainly used to make tires, rubber shoes, hoses, tapes, cables and general industrial products.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/134.jpgextended-reading:https://www.bdmaee.net/epoxy-curing-agent/extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/73.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/11extended-reading:https://www.newtopchem.com/archives/39388extended-reading:https://www.bdmaee.net/dabco-xd-103-dabco-tertiary-amine-catalyst-catalyst-xd-103/extended-reading:https://www.newtopchem.com/archives/658extended-reading:https://www.newtopchem.com/archives/44199extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/31-7.jpgextended-reading:https://www.bdmaee.net/nt-cat-mb20-catalyst-cas-68007-43-3-newtopchem/


