Pentachloronitrobenzene
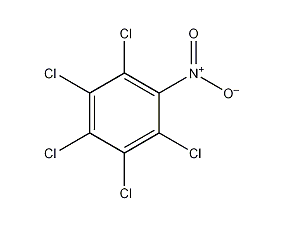
Structural formula
| Business number | 01T3 |
|---|---|
| Molecular formula | C6Cl5NO2 |
| Molecular weight | 295.33 |
| label |
Soil particles scattered, Pentachloronitrobenzene raw powder, Nitro-pentachlorobenzene, Digging raw powder, Botrilex,Chinozan,Pentagen,Terrachlor, Fungicides; aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:82-68-8
MDL number:MFCD00007065
EINECS number:201-435-0
RTECS number:DA6650000
BRN number:1914324
PubChem number:24898950
Physical property data
1. Properties: Colorless or slightly yellow crystals with a musty smell
2. Density (g/mL, 25/4℃): 1.718
3. Relative Vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 146
5. Boiling point (ºC, normal pressure): 328
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Insoluble in water, slightly soluble in alcohol, benzene, chloroform, carbon disulfide
Toxicological data
Acute toxicity:
Rat caliber LD50: 750 mg/kg; rat inhalation LC50: 1400 mg/kg;
Rat abdominal LD50: 5 mg/kg;
Mouse caliber LD50: 1400 mg/kg; mouse inhalation LC50: 2 mg/kg;
Dog caliber LD: 2500 mg/kg;
Rabbit caliber LD50: 800 mg/kg; Rabbit skin LD: >4 gm/kg;
2. Chronic toxicity/carcinogenicity: Mouse caliber TDL0: 135 gm/kg/77W-C
3. Teratogenicity
E. coli: 1 mg/disc; E. coli: 10 mg/plate; E. coli: 20 umol/L
Aspergillus: 5 umol/L
Ecological data
None yet
Molecular structure data
1. MooreEmissivity: 57.27
2. Molar volume (cm3/mol): 161.0
3. Isotonic specific volume (90.2K): 442.1
4. Surface tension (dyne/cm): 56.8
5. Polarizability (10-24cm3): 22.70
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 219
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It has stable chemical properties, is not easy to volatilize, is not easy to be hydrolyzed and oxidized, and is not affected by sunlight, temperature and pH, and has a long residual effect in the soil.
Low-toxic fungicides and medicines. Rat acute oral administration: LD501700mg/kg. Production equipment should be airtight. Operators should wear protective equipment.
Storage method
Keep sealed and protected from light
This product is packed in iron drums, with a net weight of 50kg or 100kg per drum. Store in a cool, dry place to prevent moisture. Store and transport according to general chemical regulations.
Synthesis method
Use iodine as the catalyst and chlorosulfonic acid as the solvent to react 2,4,5-trichloronitrobenzene with chlorine at 80-85°C. The dosage of solvent chlorosulfonic acid is 1-3 times the weight of chloronitrobenzene, the dosage of iodine is 0.2-0.3% of the weight of chloronitrobenzene, and chlorine is passed through to an excess of 1%. Then cool to 30°C, filter out pentachloronitrobenzene, wash with 80-90°C hot water until neutral, and dry at 70°C to obtain a product with a content of 98-99% and a yield of 94%. 2,4,5-Trichloronitrobenzene is obtained by nitration of 1,2,4 trichloronitrobenzene. The above method consumes iodine and chlorosulfonic acid, and the equipment is easy to corrode. Another production method is to use trichlorobenzene obtained from 666 non-toxic body to nitrate and chloride, which can obtain a chloronitrobenzene content of more than 70%. of original powder.
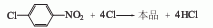
1,2,4-Trichlorobenzene is nitrated to obtain trichloronitrobenzene. Then use chlorosulfonic acid as the solvent and iodine as the catalyst, pass chlorine at 80-85°C, and obtain pentachloronitrobenzene after chlorination.
Purpose
1. Excellent soil fungicide, used as seed dressing agent and soil treatment agent, used to prevent and control cotton blight and damping-off, wheat and sorghum smut, potato scab, cabbage clubroot, and lettuce Gray mold, etc. Spraying can prevent and control rice sheath blight.
2.Protective fungicide, low toxicity, no systemic effect. It is used for seed disinfection and soil treatment. Its bactericidal mechanism is to affect the mitosis of hyphal cells. To control wheat smut and loose smut, use 50g of 40% powder to dress 100kg of seeds; to control cotton seedling diseases, use 40% powder to dress 100g of seeds; to control rapeseed For Sclerotinia sclerotiorum, use 65.6g of 40% powder mixed with 1.5-3kg of fine soil per 100 square meters, sprinkle it near the roots at the early stage of the disease; to prevent potato scab, use 65.6g of 40% powder per 100 square meters. Use 375-675g of 50% wettable powder for rice and apply it in strips in the sowing ditch.
extended-reading:https://www.cyclohexylamine.net/semi-hard-foam-catalyst-tmr-3-hard-foam-catalyst-tmr-3/
extended-reading:https://www.bdmaee.net/pc-cat-tap-amine-catalysts-trimethylamine-ethyl-piperazine-nitro/
extended-reading:https://www.bdmaee.net/cas-251-964-6/
extended-reading:https://www.bdmaee.net/butylenestannonic-acid/
extended-reading:https://www.cyclohexylamine.net/main-5/
extended-reading:https://www.newtopchem.com/archives/category/products/page/108
extended-reading:https://www.bdmaee.net/high-tin-chloride/
extended-reading:https://www.cyclohexylamine.net/dibutyltin-monooctyl-maleate-cas-25168-21-2/
extended-reading:https://www.newtopchem.com/archives/1087
extended-reading:https://www.bdmaee.net/pc-cat-dmp-catalyst-14-dimethylpiperazine-nitro/


